 |
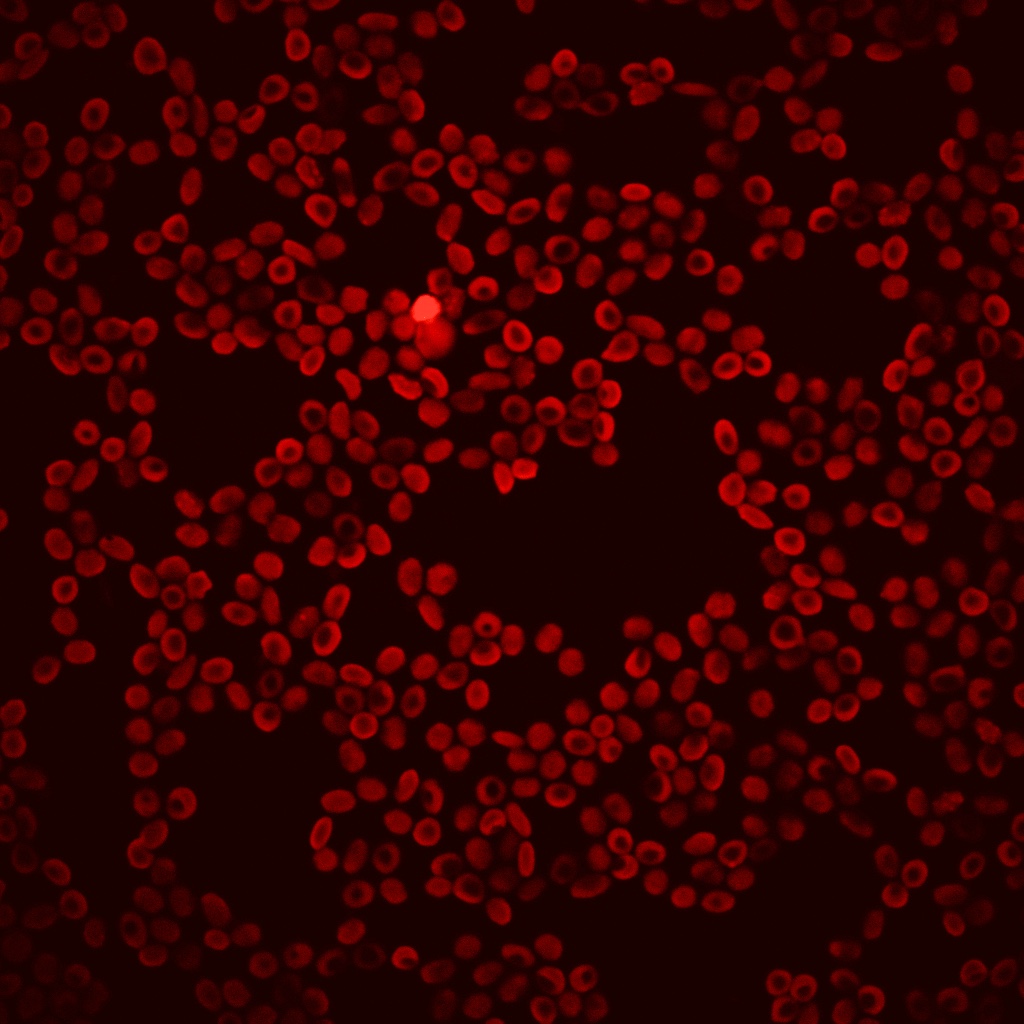
Red blood cells are the most common cell type in the human body and it can be circulated to all tissues of our body. We have established a mature in vitro red blood cell regeneration culture technology, which uses patients’autologous peripheral blood to simulate erythroid differentiation culture in vitro. By expressing the functional proteins in erythroid progenitors and generating RBCs by in vitro culture, a large number of mature RBCs expressing functional proteins were obtained in vitro. Engineering red blood cells are novel drug carriers and have great potential to be applied into disease treatment, like rare diseases, cancer, autoimmune diseases, metabolic syndromes and virus infections. |
|
The blood is rich in biomarkers, such as circulating tumor cells (CTCs), circulating free DNA (cfDNA), platelets, exosomes, etc., which have been widely used in the current liquid biopsy and early disease screening, but the mystery in blood is much more than that. Here, we defined a new biomarker in the blood, which is closely related to the individual’s DNA damage. Based on this, we have established a new type of disease early screening and individual health risk monitoring system, that have shown extremely high accuracy goes beyond standard clinical biomarkers. |
 |
|
 |
Our long-term interest is to understand how stem/progenitor cell fates are determined via coordination of multiple cellular factors and environmental signals under normal and stressed conditions. Stem/progenitor cells can respond to extrinsic and intrinsic signaling cues, and decide whether to undergo self-renewal or a differentiation division. This decision plays a key role in tissue regeneration and maintaining tissue homeostasis. We have used genetic, cell biology, molecular biology and biochemical approaches to systematically analyze mechanisms by which cells integrate extrinsic and intrinsic signals to make developmental decisions. |
|

Red blood cells are the most common cell type in the human body and it can be circulated to all tissues of our body. We have established a mature in vitro red blood cell regeneration culture technology, which uses patients’autologous peripheral blood to simulate erythroid differentiation culture in vitro. By expressing the functional proteins in erythroid progenitors and generating RBCs by in vitro culture, a large number of mature RBCs expressing functional proteins were obtained in vitro. Engineering red blood cells are novel drug carriers and have great potential to be applied into disease treatment, like rare diseases, cancer, autoimmune diseases, metabolic syndromes and virus infections.

The blood is rich in biomarkers, such as circulating tumor cells (CTCs), circulating free DNA (cfDNA), platelets, exosomes, etc., which have been widely used in the current liquid biopsy and early disease screening, but the mystery in blood is much more than that. Here, we defined a new biomarker in the blood, which is closely related to the individual’s DNA damage. Based on this, we have established a new type of disease early screening and individual health risk monitoring system, that have shown extremely high accuracy goes beyond standard clinical biomarkers.

Our long-term interest is to understand how stem/progenitor cell fates are determined via coordination of multiple cellular factors and environmental signals under normal and stressed conditions. Stem/progenitor cells can respond to extrinsic and intrinsic signaling cues, and decide whether to undergo self-renewal or a differentiation division. This decision plays a key role in tissue regeneration and maintaining tissue homeostasis. We have used genetic, cell biology, molecular biology and biochemical approaches to systematically analyze mechanisms by which cells integrate extrinsic and intrinsic signals to make developmental decisions.
In addition, by using single-cell RNA sequencing technology, we have dissected the heterogeneity within a cell population and discovered novel mechanisms underlying stem cell self-renewal. More importantly, building on our basic research, we have discovered several drugs that can retain or promote the self-renewal capacity of certain stem cells for therapeutic purposes.
Gao X*, Lee HY*, Li W, Platt RJ, Barrasa MI, Ma Q, Elmes RR, Rosenfeld MG and Lodish HF
Proc Natl Acad Sci U S A. 2017 Sep 19;114(38):10107-10112.
TGF-β inhibitors stimulate red blood cell production by enhancing self-renewal of BFU-E erythroid progenitors
Gao X, Lee HY, Lummertz Da Rocha E, Lu YF, YX Feng, Barrasa MI, Cahan P, Li H, Daley GQ, Lodish HF
Blood. 2016 Dec 8;128(23):2637-2641. doi: 10.1182/blood-2016-05-718320.
Citrobacter rodentium NleB Protein Inhibits Tumor Necrosis Factor (TNF) Receptor-associated Factor 3 (TRAF3) Ubiquitination to Reduce Host Type I Interferon Production
Gao X, Pham TH, Feuerbacher LA, Chen K, Hays MP, Singh G, Rueter C, Guerrero RH, Hardwidge PR
Journal of Biological Chemistry. 2016 Jul 7. pii: jbc.M116.738278.
PPAR-α and glucocorticoid receptor synergize to promote erythroid progenitor self-renewal
Lee HY*, Gao X*, Barrasa MI, Li H, Elmes RR, Peters LL and Lodish HF
Nature. 2015 Jun 25;522(7557):474-7. doi: 10.1038/nature14326. Epub 2015 May 11.
NleB, a bacterial effector with glycosyltransferase activity, targets GAPDH function to inhibit NF-κB activation
Gao X, Wang X, Pham T, Feuerbacher LA, Lubos ML, Huang M, Olsen R, Mushegian A, Slawson C and Hardwidge PR
Cell Host Microbe. 2013 Jan 16;13(1):87-99. doi: 10.1016/j.chom.2012.11.010.
Escherichia coli virulence protein NleH1 interaction with the v-Crk sarcoma virus CT10 oncogene-like protein (CRKL) governs NleH1 inhibition of the ribosomal protein S3 (RPS3)/nuclear factor κB (NF-κB) pathway
Pham T, Gao X, Singh G and Hardwidge PR
Journal of Biological Chemistry. 2013 Nov 29;288(48):34567-74. doi: 10.1074/jbc.M113.512376.
Ribosomal protein s3: a multifunctional target of attaching/effacing bacterial pathogens
Gao X and Hardwidge PR
Frontiers in Microbiology. 2011 Jun 27;2:137. doi: 10.3389/fmicb.2011.00137. eCollection 2011.
IKKβ phosphorylation regulates RPS3 nuclear translocation and NF-κB function during infection with Escherichia coli strain O157:H7
Wan F, Weaver A, Gao X, Bern M
Nature Immunology. 2011 Apr;12(4):335-43. doi: 10.1038/ni.2007. Epub 2011 Mar 13.
Bacterial effector binding to ribosomal protein s3 subverts NF-kappaB function
Gao X, Wan F, Mateo K, Callegari E, Wang D, Deng W, Puente J, Li F, Chaussee MS, Finlay BB, Lenardo MJ and Hardwidge PR
PLoS Pathogens. 2009 Dec;5(12):e1000708. doi: 10.1371/journal.ppat.1000708.


